Final Answer:
The mass of vanadium
 in
in
 atoms is approximately
atoms is approximately
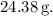
Step-by-step explanation:
To calculate the mass of vanadium
 in
in
 atoms, we use the molar mass of vanadium, which is the mass of one mole of vanadium atoms. The molar mass of vanadium is
atoms, we use the molar mass of vanadium, which is the mass of one mole of vanadium atoms. The molar mass of vanadium is

First, calculate the number of moles
 in
in
 atoms using Avogadro's number
atoms using Avogadro's number

![\[ n = (4.18 * 10^(23))/(6.022 * 10^(23)) \]](https://img.qammunity.org/2024/formulas/physics/high-school/wo443zkmw8p1nsjilz9im1q4v5yu32f6a6.png)
Next, use the molar mass to find the mass
 in grams:
in grams:
![\[ m = n * \text{Molar Mass} \]](https://img.qammunity.org/2024/formulas/physics/high-school/dnadfiwefq693v5lgen1q9i8gbszpl1gfv.png)
Substitute the values:
![\[ m = (4.18 * 10^(23))/(6.022 * 10^(23)) * 50.94 \]](https://img.qammunity.org/2024/formulas/physics/high-school/ezss879bqqo2773wym68s533j9eg3zjth0.png)
Calculating this expression gives the mass of vanadium in grams. Therefore, the mass of
 in
in
 atoms is approximately
atoms is approximately
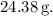
Understanding the concept of molar mass and Avogadro's number is essential in converting between the microscopic scale of atoms or molecules and the macroscopic scale of grams or moles. This calculation is a fundamental step in chemistry for determining the quantity of a substance based on its atomic or molecular composition.