Matter and Change - Chemical reaction - Reactants and products.
A chemical reaction is a process in which some substances (reactants) change into different substances (products).
Answer:
The reactants are present in the start of the chemical reaction while products are present at the end of the chemical reaction.
A reaction rate is the change in concentration of a reactant or product with time, and it is affected by the concentration of the reactants and products. An increase in the concentration of one or more reactants results in an increase of the reaction rate.
In this case we have the following net ionic chemical reaction:
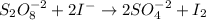
We can see that the reactants are:
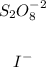
So an increase in the concentration of any of them would increase the rate of the reaction.
So the answer is B and C.
B: Iodide ions (I-)
C:Persulfate ions (S2O8-2)