ANSWER
The energy produced per mole of fuel is 808 KJ
The energy produced per tank of fuel is 1082.14KJ
Step-by-step explanation
Given that;
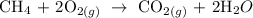
Follow the steps below to find the energy produced per mole
Step 1; Calculate the heat of formation of the fuel
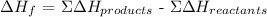
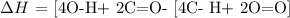
In the table provided,
C- H = 413 KJ/mol
O=O = 495 KJ/mol
C=O = 799 KJ/mol
O-H = 463 KJ/mol
Substitute the given data into the above formula
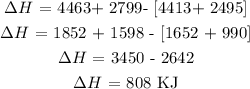
Therefore, the energy produced per mole of fuel is 808 KJ
The next step is to find the energy produced per tank of fuel
Recall, that 1 mole of gas is 22.4L
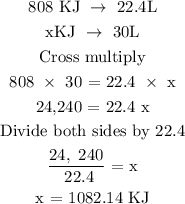
Therefore, the energy produced per tank of fuel is 1082.14KJ