Part A:
Given:
The volume of acetone, V=17.96 mL.
We know, the density of acetone is D=0.7900 g/mL.
Now, the mass of acetone is,
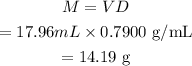
Therefore, the mass in grams of acetone upto four significant figures is 14.19g.
Part B.
Given:
The mass of acetone, M=7.40 g.
The volume is,
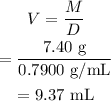
Therefore, the volume in mL upto three significant figures is is 9.37 mL