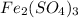Answer: The compound is
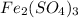
Step-by-step explanation:
Molar mass is defined as the mass in grams of 1 mole of a substance.
S.I Unit of Molar mass is gram per mole and it is represented as g/mol.
A. 1 mole of
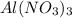 weighs = 26.98(1)+14.01(3)+15.99(9) =212.99 g/mol
weighs = 26.98(1)+14.01(3)+15.99(9) =212.99 g/mol
B. 1 mole of
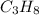 weighs = 12.01(3)+1.007(8) = 44.1 g/mol
weighs = 12.01(3)+1.007(8) = 44.1 g/mol
C. 1 mole of
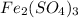 weighs = 55.84(2)+32.06(3)+15.99(12) = 399.91 g/mol
weighs = 55.84(2)+32.06(3)+15.99(12) = 399.91 g/mol
D. 1 mole of
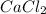 weighs = 40.07(1)+35.5(2)= 110.98 g/mol
weighs = 40.07(1)+35.5(2)= 110.98 g/mol
Thus the compound is