Here, the pressure the gas exerts varies inversely with the volume.
Where the constant, k = temperature of the gas
Let p represent the pressure the gas exerts
Let v represent the volume.
Since the relationship here is an inverse variation, we have the formula:
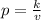
Given:
v = 76
p = 16
Let's solve for k:
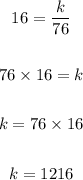
Now, let's find the volume when the pressure is 64.
Substitute 1216 for k and 64 for p, to find the volume:
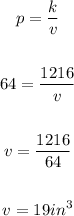
Therefore, when the pressure is 64 pounds per square inch, the volume will be 19 in. cubed.
ANSWER:
