Answer:
The final equilibrium temperature of the tin-water mixture is approximately 18.468 °C
Step-by-step explanation:
The parameters of heat energy transfer from the tin to the water are given as follows;
The mass of the sample of tin, m₁ = 0.225 kg
The initial temperature of the tin, T₁ = 97.5 °C
The mass of the water into which the tin is dropped, m₂ = 0.115 kg
The initial temperature of the water, T₂ = 10.0 °C
The specific heat capacity of tin, c₁ = 230 J/(kg·°C)
The specific heat capacity of water, c₂ = 4,200 J/(kg·°C)
Let 'T' represent the final equilibrium temperature of the tin-water mixture, we have;
The heat lost by the tin, ΔQ
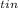 = The heat gained by the water ΔQ
= The heat gained by the water ΔQ
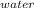
∴ ΔQ
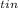 = ΔQ
= ΔQ
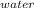
Where;
ΔQ
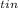 = m₁·c₁·(T₁ - T)
= m₁·c₁·(T₁ - T)
ΔQ
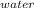 = m₂·c₂·(T - T₂)
= m₂·c₂·(T - T₂)
By substitution, we have;
ΔQ
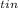 = 0.225 kg × 230 J/(kg·°C) × (97.5°C - T)
= 0.225 kg × 230 J/(kg·°C) × (97.5°C - T)
ΔQ
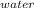 = 0.115 kg × 4,200 J/(kg·°C) × (T - 10.0°C)
= 0.115 kg × 4,200 J/(kg·°C) × (T - 10.0°C)
From ΔQ
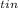 = ΔQ
= ΔQ
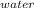 , we have;
, we have;
0.225 kg × 230 J/(kg·°C) × (97.5°C - T) = 0.115 kg × 4,200 J/(kg·°C) × (T - 10.0°C)
∴ 5,045.625 J - 51.75 J/°C × T = 483 J/°C × T - 4,830 J
5,045.625 J + 4,830 J = 534.75 J/°C × T
∴ 534.75 J/°C × T = 9,875.625 J
T = 9,875.625 J/(534.75 J/°C) = 18.4677419 °C ≈ 18.468 °C
The final equilibrium temperature of the tin-water mixture, T ≈ 18.468 °C.