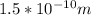Answer:

Step-by-step explanation:
Given
 -- radius of hydrogen atom
-- radius of hydrogen atom
See attachment
Required
Determine the radius of magnesium atom (R)
From the attachment, the ratio of a hydrogen atom to a magnesium atom is:
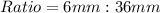
Simplify
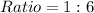
Represent the radius as ratio:
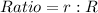
Substitute


Equate both ratios
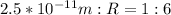
Express as fraction
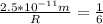
Cross Multiply


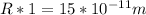

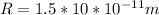
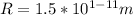

Hence, the radius of the magnesium atom is: