Answer:
B. The atom has two more electrons than an atom of oxygen.
Step-by-step explanation:
In Chemistry, electrons can be defined as subatomic particles that are negatively charged and as such has a magnitude of -1.
Valence electrons can be defined as the number of electrons present in the outermost shell of an atom. Valence electrons are used to determine whether an atom or group of elements found in a periodic table can bond with others. Thus, this property is typically used to determine the chemical properties of elements.
Oxygen has a total number of eight (8) electrons while neon has total number of ten (10) electrons. Therefore, an atom of oxygen is able to gain (receive) two (2) more electrons in order to have the same electron arrangements as the noble gas i.e an atom of neon with an atomic number of 10.
This ultimately implies that, an
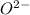 atom contains the same number of electrons as an atom of neon.
atom contains the same number of electrons as an atom of neon.
Hence, a neon atom has two more electrons than an atom of oxygen.
Additionally, Neon is used in lighting advertising displays.