Answer: The enthalpy change for this reaction is, -803 kJ
Step-by-step explanation:
The balanced chemical reaction is,
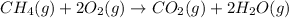
The expression for enthalpy change is,
![\Delta H=\sum [n* \Delta H_f(product)]-\sum [n* \Delta H_f(reactant)]](https://img.qammunity.org/2022/formulas/chemistry/high-school/ki3v3y7u11m1ff8fhba8jdrhiw2jcbq6df.png)
![\Delta H=[(n_(CO_2)* \Delta H_(CO_2))+(n_(H_2O)* \Delta H_(H_2O))]-[(n_(O_2)* \Delta H_(O_2))+(n_(CH_4)* \Delta H_(CH_4))]](https://img.qammunity.org/2022/formulas/chemistry/high-school/xqjw2jxvxche1o64hkm8yc4z1icbedyd2t.png)
where,
n = number of moles
Now put all the given values in this expression, we get
![\Delta H=[(1* -394)+(2* -242]-[(2* 0)+(1* -75)]](https://img.qammunity.org/2022/formulas/chemistry/high-school/2yfx4eip63jz49e0nkb2mk63mp08k197st.png)

Therefore, the enthalpy change for combustion of methane is, -803 kJ