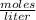Answer:
The molarity is 0.0174
Step-by-step explanation:
Molarity (M) or Molar Concentration is the number of moles of solute that are dissolved in a certain volume.
The molarity of a solution is calculated by dividing the moles of the solute by the volume of the solution.
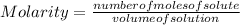
Molarity is expressed in units
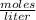 .
.
In this case, being the molar mass of compound Pb(C₂H₃O₂)₄ equal to 443.38 g / mol, then the following rule of three can be applied: if 443.38 grams are contained in 1 mol, 2.7*10⁻² grams in how many moles they are present?
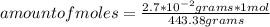
amount of moles= 6.09*10⁻⁵ moles
Being 1,000 mL= 1 L, then 3.5 mL= 3.5*10⁻³ L
So:
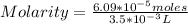
Solving:
Molarity=0.0174
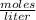
The molarity is 0.0174