ANSWER
The number of moles of NaOH is 432 moles
Step-by-step explanation
Given information
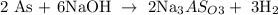
The number of moles of As = 144 moles
Let x represents the number of moles of NaOH
From the above reaction, you will see that 2 moles of As react with 6 moles of NaOH
The next step is to find the number of moles of NaOH using a stoichiometry ratio
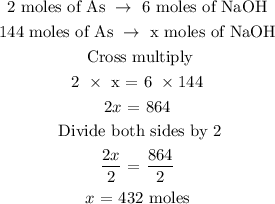
Hence, the number of moles of NaOH is 432 moles