Answer
Molecules of water = 1.204 x 10^24
Hydrogen atoms = 2.408 x 10^24
Oxygen atoms = 1.204 x 10^24
Explanation:
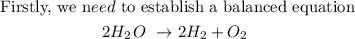
From the equation,
We have 2 moles of water
2 moles of hydrogen
1 mole of oxygen
Mole = no of molecules / Avogadro's number
No of molecules = mole x Avogadro's number
For water
No of molecules = 2 * 6.02 x 10^23
No of molecues = 12.04 x 10^23
No of molecules = 1,204 x 10^24
Hence, water has 1.204 x 10^24 molecules
From the chemical equation,
We have two moles of Hydrogen
Hence, the number of hydrogen atoms can be calculated as'
2 * 1.204 x 10^24
2.408 x 10^24
Hence, we have 2.408 x 10^24 of Hydrogen atoms in 2 moles of water