Answer
38.8 mL
Step-by-step explanation
Given:
Molarity of KOH, Cb = 0.623 M
Volume of H2SO4, Va = 23.5 mL = 0.0235 L
Molarity of H2SO4, Ca = 0.514 M
What to find:
The volume of KOH required to neutralize the acid.
Step-by-step Solution:
Step 1: Balance the equation for the reaction.
The balanced equation for the reaction is:
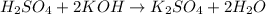
Step 2: Calculate the volume of the base, KOH.
The volume of KOH can be determine using:

From the balanced equation, na = 1 and nb = 2. so putting the values of the given parameters into the above formula, we have:

Therefore, the volume of 0.623 M KOH is required to neutralize 23.5 mL if a 0.514 M H2SO4 solution = 38.8 mL