Answer:
0.63grams
Explanations
The balanced reaction between Zinc and hydrochloric acid is given as:
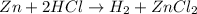
Given the following parameters
Mass of Zinc = 20.5 grams
Determine the moles of zinc
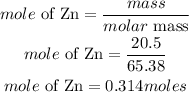
According to stoichiometry 1 mole of Zinc produces 1 mole of hydrogen gas.The moles of hydrogen that was produced will be 0.314moles
Determine the mass of hydrogen gas
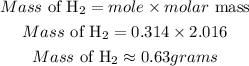
Hence the mass of hydrogen gas that will be produced is 0.63grams