Given,
The initial pressure of the gas, P₁=P N/m²
The initial temperature of the gas, T₁=27 °C=300.15 K
The final temperature of the gas, T₂=77 °C=350.15 K
Let the new pressure of the gas be P₂
From Gay-Lussac's law,
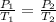
On substituting the known values,
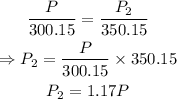
Therefore the new pressure will be 1.17 times the initial pressure, i.e., 1.17 P