With the given equation we cannot proceed to calculate the moles of H2 that are needed, since it is not balanced. We must balance it first. The balanced equation will be one that has the same number of atoms of each element on each side of the reaction. Let's start with the original equation:
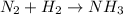
We see that there are two nitrogens in the reactants, so we put the 2 in front of the NH3 molecule:
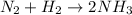
Now we have 6 hydrogens in the products, to have the same amount in the reactants we put the coefficient 3 in front of the H2 molecule:
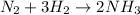
Now, the equation is balanced and we can proceed.
The ratio H2 to NH3 is 3/2, if we have 9 moles of NH3 we will need:
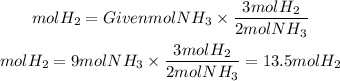
We will need 14mol of H2 to produce 9 moles of NH3