Answer:
C: Claim the answer.
No, 100 g of NH4Cl will not form a saturated solution with 200 g of water.
E: Evidence.
According to table G and by doing a rule of three, we obtained 124 g of NH4Cl and the question is asking if a solution with 100 g of NH4Cl with 200 g of water.
R: Reasoning.
According to the result that we obtained from the rule of three, we need 124 g of NH4Cl to form a saturated solution with 200 g of water, so 100 g of NH4Cl with 200 g of water will form an unsaturated solution.
Step-by-step explanation:
First, let's review the concept of a saturated solution: A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving.
You can see in the graph that at 70 °C, we can dissolve approximately 62 g of NH4Cl in 100 g of water to form a saturated solution. But, we want to confirm if 100 g of NH4Cl can be dissolved in 200 g of water to form a saturated solution, so let's state a rule of three:
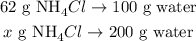
The calculation of this rule of three would be:
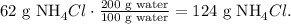
To form a saturated solution of NH4Cl with 200 g of water, we will need 124 g of NH4Cl. As we obtained 124 g of NH4Cl and the question is asking if a solution with 100 g of NH4Cl with 200 g of water will form a saturated solution would be wrong because this amount doesn't reach the maximum of solute. This would be an unsaturated solution (an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved).
C: Claim the answer.
No, 100 g of NH4Cl will not form a saturated solution with 200 g of water.
E: Evidence.
According to table G and by doing a rule of three, we obtained 124 g of NH4Cl and the question is asking if a solution with 100 g of NH4Cl with 200 g of water.
R: Reasoning.
According to the result that we obtained from the rule of three, we need 124 g of NH4Cl to form a saturated solution with 200 g of water, so 100 g of NH4Cl with 200 g of water will form an unsaturated solution.