The formula for density d is given by
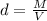
where M is the mass and V is the volume.
Lets start !
- Mercury
![d=(3000)/(220.58)=13.6\text{ }\frac{gr}{\operatorname{cm}\mleft\lbrace^3\mright?}]()
- Iron
![d=(750)/(96.2)=7.79\frac{gr}{\operatorname{cm}\mleft\lbrace^3\mright?}]()
- Lead
![d=(1800.9)/(159.3)=11.31\frac{gr}{\operatorname{cm}\mleft\lbrace^{}^3\mright?}]()
- Gold
![d=(85)/(44)=1.93\frac{gr}{\operatorname{cm}\mleft\lbrace^3\mright?}]()
- Aluminum
![d=(150.12)/(55.6)=2.7\frac{gr}{\operatorname{cm}\lbrace^3}]()
- Copper
![d=(275)/(30.89)=8.9\frac{gr}{\operatorname{cm}\lbrace^3}]()
- Marble
![d=(12350)/(4750)=2.6\frac{gr}{\operatorname{cm}\lbrace^3}]()
- Wood
![d=(5000)/(7692)=0.65\frac{gr}{\operatorname{cm}\lbrace^3}]()
- Concrete
![d=(15250)/(6630.4)=2.3\frac{gr}{\operatorname{cm}\lbrace^3}]()
Now, lets go to organize the density from least dense to most dense. From our previous result, the organized table is:
1. Wood
2. Gold
3. Concrete
4. Marble
5. Aluminum
6. Iron
7 Copper
8. Lead
9. Mercury
Which materials will sink in water? Water density is about 1 gr/cm^3, so more dense materials will sink in water. In our case, those materials are: Gold, Concrete, Marble, Aluminum, Iron, Copper, Lead and Mercury