299.7g of H2O will be produced.
1st) With the balanced chemical equation and the molar mass of CH4 and H2O, we can calculate the amount of H2O that is produced by stoichiometry:
- CH4 molar mass: 16g/mol
- H2O molar mass: 18g/mol
According to the balanced equation, 16g of CH4 will produce 36g of H2O (2 x 18g).
2nd) Now, with a mathematical rule of three we can calculate the grams of water that will be produced from 133.2 grams of CH4:
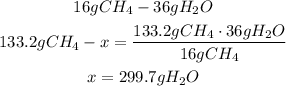
Finally, 299.7g of H2O will be produced from 133.2 grams of CH4.