Answer:
157.096grams
Explanations:
The formula for calculating the molarity of a solution is expressed as:
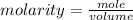
Given the following parameters
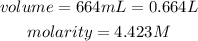
Determine the mole of the solution
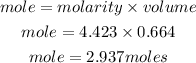
Determine the mass of NH4Cl
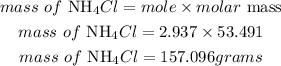
Hence the mass in grams of ammonium chloride is 157.096grams