Answer:
Explanations:
The formula for calculating the exponential decay of an element is expressed as:
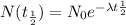
No is the original sample
N(t) is the final sample after time "t"
λ is the decay constant
t is the time
Given the following parameters
No = 4.0grams
t = 30 seconds
Determine the decay constant
![undefined]()