b)
Concentartion of hydroxide ion:
pOH = 14 - 4.74
pOH = 9.26
[OH-] = 10^-9.26 = 5.5x10^-10
Therefore: The solubility of Al(OH)3 in this buffer is then calculated from its solubility product expressions:
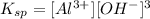
The molar solubility in buffer = [Al^3+] = Ksp/[OH-] = 2x10^-32
2x10^-32 = Al (5.5x10^-10)^3
Al = 1.2x10^-4 M