To find the temperature of the gas we can use the ideal gas law:

where P is the pressure, V is the volume, n is the number of moles of the gas, R is the unirversal gas constant (0.08205746 L atm/ mol K) and T is the temperature.
Plugging the values given into the equation we have:
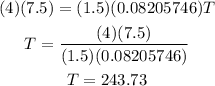
Therefore the temperature is 243.73 K