First, we have to remember the molarity formula:
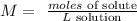
Part 1:
In this case, our solute is sodium nitrate (NaNO3), and we have the mass dissolved in water, then we have to convert grams to moles. For that, we need the molecular weight:
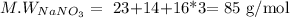
Then, we calculate the moles present in the solution:
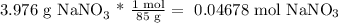
Now, we have the necessary data to calculate the molarity (with the solution volume of 200 mL):
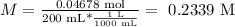
The molarity of this solution equals 0.2339 M.
Part 2:
In this case, we have the same amount (in moles and mass) of sodium nitrate, but a different volume of solution, then we only have to change it:
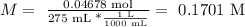
So, the molarity of this solution is 0.1701 M.