Answer: Considering the information provided, the moalrity of the acid is 1 M
Step-by-step explanation:
The question requires us to calculate the final molarity of the acid, given the volume and concentration of NaOH used.
The following information was provided by the question:
initial amount of NaOH = 70 mL
final amount of NaOH = 45 mL
molarity of NaOH = 1 M
Considering that the question refers to a titration procedure where NaOH is used to neutralize an acid with only one H+ ion (such as HCl), we can say that the reaction between the base and the acid would be as it follows:

(note that "HA" was used as a generic notation for the acid).
At the neutralization point, where the titration ends, we can say that the number of moles of acid (H+) and base (OH-) are the same. Thus, we can write:
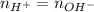
And we can calculate the number of moles of base from its molarity (1 M) and volume used (70 mL - 45 mL = 25 mL), according to the following equation:
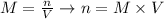
Combining these two equations and applying the values provided by the question, we can calculate the number of moles of acid:,
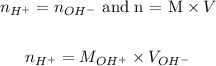
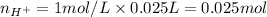
Therefore, the number of moles of acid is 0.025 moles.
As the question does not provide the volume of acid used, we'll assume that the volume in which the acid is contained is 0.025 L (the volume of NaOH added). Thus, the molarity of acid can be calculated as:
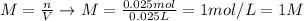
Therefore, the moalrity of the acid is 1 M.