Final answer:
The given problem involves determining the values for
 in the chemical equation
in the chemical equation
 based on the provided yield coefficient
based on the provided yield coefficient
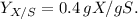
Options A, B, C and D are none of the correct ones
Step-by-step explanation:
To determine the values for
 , we can use the stoichiometry of the given chemical equation and the yield coefficient
, we can use the stoichiometry of the given chemical equation and the yield coefficient
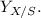
The given equation is:
![\[ C_3H_6O_3 + a \, O_2 + b \, NH_3 \rightarrow c \, C_5H_7NO_2 + d \, H_2O + e \, CO_2 \]](https://img.qammunity.org/2024/formulas/biology/high-school/qm0x6ts3mwcyh8pe8jt5mc146xwcob7tsi.png)
The yield coefficient
 is defined as the amount of biomass
is defined as the amount of biomass
 produced per unit of substrate
produced per unit of substrate
 consumed. In this case,
consumed. In this case,
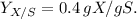
The molecular weight of \(C_3H_6O_3\) is approximately 90 g/mol.
Now, let's balance the equation using the given options:
a.
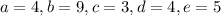
![\[\text{C}_3\text{H}_6\text{O}_3 + 4 \, \text{O}_2 + 9 \, \text{NH}_3 \rightarrow 3 \, \text{C}_5\text{H}_7\text{NO}_2 + 4 \, \text{H}_2\text{O} + 5 \, \text{CO}_2\]](https://img.qammunity.org/2024/formulas/biology/high-school/toznxoah2u430kc7vcc2496qvfu7rgsykk.png)
Now, let's check if the yield coefficient
 is satisfied:
is satisfied:
![\[ Y_(X/S) = \frac{\text{moles of } C_5H_7NO_2}{\text{moles of } C_3H_6O_3} = \frac{3 * \text{MW}_(C_5H_7NO_2)}{\text{MW}_(C_3H_6O_3)} = (3 * 113)/(90) \]](https://img.qammunity.org/2024/formulas/biology/high-school/4bkudwvztqbc3lacarz7myy5hi2z98cehz.png)
However, this does not equal 0.4, so option a is not correct.
b.
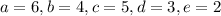
![\[\text{C}_3\text{H}_6\text{O}_3 + 6 \, \text{O}_2 + 4 \, \text{NH}_3 \rightarrow 5 \, \text{C}_5\text{H}_7\text{NO}_2 + 3 \, \text{H}_2\text{O} + 2 \, \text{CO}_2\]](https://img.qammunity.org/2024/formulas/biology/high-school/7o1vcgt8ft0ygp9wwxib09b707z4iycj82.png)
Now, let's check if the yield coefficient
 is satisfied:
is satisfied:
![\[ Y_(X/S) = \frac{\text{moles of } C_5H_7NO_2}{\text{moles of } C_3H_6O_3} = \frac{5 * \text{MW}_(C_5H_7NO_2)}{\text{MW}_(C_3H_6O_3)} = (5 * 113)/(90) \]](https://img.qammunity.org/2024/formulas/biology/high-school/rauukvb2lwrwrruc5l0g8mnyonnvhr9jzi.png)
This value does not equal 0.4 either, so option b is not correct.
c.
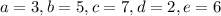
![\[\text{C}_3\text{H}_6\text{O}_3 + 3 \, \text{O}_2 + 5 \, \text{NH}_3 \rightarrow 7 \, \text{C}_5\text{H}_7\text{NO}_2 + 2 \, \text{H}_2\text{O} + 6 \, \text{CO}_2\]](https://img.qammunity.org/2024/formulas/biology/high-school/atq3walgk7j30pnzyv007d6ttfznl3alkw.png)
Now, let's check if the yield coefficient
 is satisfied:
is satisfied:
![\[ Y_(X/S) = \frac{\text{moles of } C_5H_7NO_2}{\text{moles of } C_3H_6O_3} = \frac{7 * \text{MW}_(C_5H_7NO_2)}{\text{MW}_(C_3H_6O_3)} = (7 * 113)/(90) \]](https://img.qammunity.org/2024/formulas/biology/high-school/s614w6ek72l7nlvhlpnqvc38zrmsky8l6q.png)
This value does not equal 0.4 either, so option c is not correct.
d.
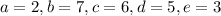
![\[\text{C}_3\text{H}_6\text{O}_3 + 2 \, \text{O}_2 + 7 \, \text{NH}_3 \rightarrow 6 \, \text{C}_5\text{H}_7\text{NO}_2 + 5 \, \text{H}_2\text{O} + 3 \, \text{CO}_2\]](https://img.qammunity.org/2024/formulas/biology/high-school/lwg7m8fqomvenhb8k16d1irrsif3m75l3s.png)
Now, let's check if the yield coefficient
 is satisfied:
is satisfied:
![\[ Y_(X/S) = \frac{\text{moles of } C_5H_7NO_2}{\text{moles of } C_3H_6O_3} = \frac{6 * \text{MW}_(C_5H_7NO_2)}{\text{MW}_(C_3H_6O_3)} = (6 * 113)/(90) \]](https://img.qammunity.org/2024/formulas/biology/high-school/i7qkm4t1cri2293jomw0vzsme93vpo9wp2.png)
This value does not equal 0.4 either, so option d is not correct.
Options A, B, C and D are none of the correct ones