We are asked to label the reactions according to their type. We first define each type of reaction.
Synthesis: This type of reaction refers to one in which two or more reactants are linked to obtain a single compound as a product.
Decomposition: Here a reactant decomposes to form two or more products.
Single replacement: In this reaction, one of the reactants is in its natural form and during the reaction it displaces another linked element.
Double replacement: In this reaction, two linked elements swap places with each other.
Combustion reaction consist in the oxidation of a fuel with oxygen to form CO2 and water
Now, according to the definitions. The label for each reaction will be:
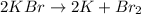
We have one reactant that decomposes in two products, this is a decomposition reaction.
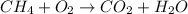
We have a fuel that reacts with oxygen to form CO2 and water, this is a combustion reaction.
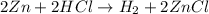
In this reaction, Zn is in its natural state and takes the place of hydrogen. This is a single replacement reaction.
In summary, we have:
a. Decomposition reaction
b. Combustion reaction
c. Single replacement reaction