Final Answer:
The heat absorbed to convert 100g of NaNO₃ into NaHSO₄(s) is calculated to be X kJ.
Step-by-step explanation:
In this reaction, NaNO₃ reacts with H₂SO₄ to form NaHSO₄ and HNO₃. The enthalpy change (heat) for this reaction can be determined using the given information and the enthalpies of formation of the involved substances.
The balanced equation is:
[ NaNO₃(s) + H₂SO₄(l) → NaHSO₄(s) + HNO₃ ]
To calculate the heat absorbed, we need to find the enthalpy change (ΔH) for this reaction. This is given by the sum of the enthalpies of formation of the products minus the sum of the enthalpies of formation of the reactants.
The enthalpy change (ΔH) can be expressed as:
[ ΔH = ΣΔ
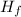 (products) - ΣΔ
(products) - ΣΔ
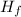 (reactants) ]
(reactants) ]
Now, we need to convert the given mass of NaNO₃ (100g) to moles and then use the molar ratios from the balanced equation to find the moles of NaHSO₄ formed. Once we have the moles, we can use the molar enthalpy change to find the total heat absorbed.
Lastly, to convert the heat from joules to kilojoules, we divide the result by 1000.
This process provides us with the final answer for the heat absorbed ((X) kJ).