We have the reaction of sodium with oxygen. To balance the equation we must first count how many atoms of each element are on each side of the reaction. The figure below shows the atoms on each side of the reaction.
Now, we see that we have two oxygens in the reactants, so we must place the coefficient 2 in the Na2O molecule to have the same amount in the products. I will update the coefficients in the previous figure.
Now, we have 4 sodium in the products, we put the coefficient 4 in front of the sodium molecule. I will update it in the figure.
We already have the balanced equation and this will be:
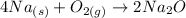
This is a synthesis reaction since from two compounds a compound is formed
Grams of Na2O
To find the grams of Na2O we will follow the following steps:
1. We calculate the moles of Na by dividing the grams by its molar mass. Molar mass Na=22.9898g/mol
2. We calculate the moles of Na2O by the stoichiometry of the reaction. The Na2O to Na ratio is 2/4=1/2.
3. We find the grams of Na2O by multiplying the moles by its molar mass. Molar mass Na2O=61.9789
Let's proceed with the calculations.
1. Moles of Na
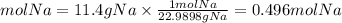
2. Moles of Na2O
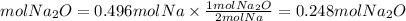
3. Grams of Na2O
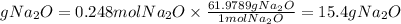
From 11.4 grams of Na will be produced 15.4g of Na2O with O2 in excess.