Answer:
Number of protons in Carbon - 14 atom
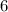
Step-by-step explanation:
The atomic number of carbon 14 atom is
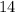
Number of neutrons in the nucleus of Carbon-14 atom
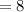
Atomic number is equal to the sum of neutrons and protons
Number of protons in Carbon - 14 atom
