Answer:
So, the combustion of 100.0g of propane produces 27.3 g of carbon dioxide.
Step-by-step explanation:
First, what we will do is balance the equation so that the atoms in the reactants match the atoms in the products.
We start by balancing the carbon. We have 3 carbon atoms on the reactant side, so we will place the coefficient 3 on the reactant side of the carbon dioxide molecule.
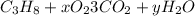
For hydrogen we have 8 atoms in the reactants, we place the coefficient 4 in front of the water molecule to match.
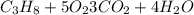
Finally, we have 10 oxygen in the products. To match the number of oxygen atoms we will place the coefficient 5 in front of the O2 molecule.
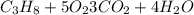
So, for each molecule of propane, we will obtain 3 molecules of carbon dioxide (CO2). We will calculate now the number of moles of propane. We will use the mass molar of propane.
Mass molar of propane = 44.1 g/mol
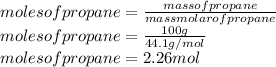
So, we will multiply the moles of propane by 3 to obtain the moles of dioxide carbon.
Moles of CO2 = 2.26 mol x 3 = 6.80 mol of CO2
We already we the moles of CO2, now to convert these moles in mass we will use the molar mass of the CO2.
Molar mass of CO2 = 44.01 g/mol
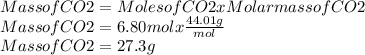
So, the combustion of 100.0g of propane produces 27.3 g of carbon dioxide.