Answer:
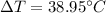
Step-by-step explanation:
Given that,
Heat measured, Q = 81500 J
Mass of water, m = 0.5 kg
The specific heat of water is 4,184 J/kg °C
We need to find the change in temperature. The heat measured is given by :
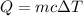
Where
 is the change in temperature
is the change in temperature
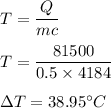
So, the change in temperature is
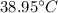 .
.