The question requires us to write the chemical reaction equation that represents the basic equilibrium for ammonia (NH3).
A general basic equilibrium equation can be written as:
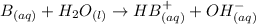
where the substance B "accpets" a H+ ion and the reaction produces OH- ions as product.
Considering the information above, we can write the following basic equation for NH3:
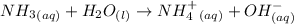
where ammonia receives an H+ ion, forming NH4+ ion and OH- ions.