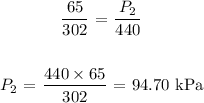Answer:
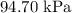
Step-by-step explanation:
Here, we want to get the new pressure
According to the pressure law, temperature and pressure are directly proportional
Mathematically, we have that as:
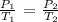
where:
P1 is the initial pressure which is 65 KPa
P2 is the final pressure that we want to calculate
T1 is the initial temperature which we will convert to Kelvin by adding 273K:
We have T1 as 29 + 273 = 302K
T2 is the final temperature which is also 167 + 273 = 440K
Substituting the values, we have it that: