Answer:
The mass of 4.76 moles lithium phosphate is 551.16 grams.
Step-by-step explanation:
Given that,
Number of moles, n = 4.76
We need to find the mass of 4.76 moles of lithium phosphate.
The chemical formula of lithium phosphate is Li₃PO₄.
Mass of Li₃PO₄ will be :
Li₃PO₄ = 3(6.941) + 30.97+4(16)
= 115.79 g/mol
The number of moles is given by :
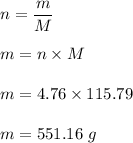
So, the mass of 4.76 moles lithium phosphate is 551.16 grams.