Answer:
pH = 7.14.
Step-by-step explanation:
Hello there!
In this case, since the pH of a solution is computed via the negative logarithm of the concentration of hydrogen ions:
![pH=-log([H_3O^+])](https://img.qammunity.org/2022/formulas/chemistry/college/pru7mfv3zsxzqm3kqb7lay44a0zk828vd1.png)
Therefore, we plug in the given concentration to obtain:
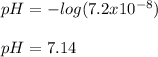
Which is a nearly-neutral solution.