The difference between the boiling points is the subtraction between them. The two boiling points are given in mixed fractions, to subtract them we need to subtract the integer part and the fractional part separately. With this in mind let's solve the problem:
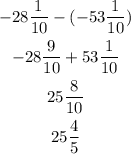
The correct answer is the option "B".