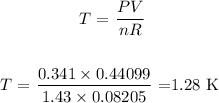Answer:
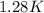
Step-by-step explanation:
Here, we want to get the temperature of the gas
We can use the ideal gas equation here
Mathematically, we have this as:
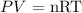
P is the pressure which is given as 34.56 kPa
V is the volume which is given as 440.99 mL
R is the molar gas constant which is 0.08205 L atm/mol k
n is the number of moles which is given as 1.43 mol
We need to convert the pressure to the correct unit (considering the molar gas consnat value in atm)
1 kPa = 0.00986923 atm
34.56 kPa will be = 34.56 * 0.00986923 = 0.341 atm
We need to convert the volume to Liters by dividing by 1000
We have that as 440.99/1000 = 0.44099 L
Finally,we rewrite the equation in terms of temperature and substitute the values as follows: