We can solve this question by using the principle of calorimetry.
The density of water is 1000 kg/m^3
According to the principle of calorimetry,
Heat lost by hot body = Heat gained by the cold body.
This principle is based on conservation of energy.
Let assume that the final temperature is t(f).
So,
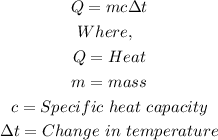
For two liter of water the final temperature is t(f) and initial temperature is 30°celcius,
So here the water will lose heat because it is at higher temperature,
![\begin{gathered} Heat\text{ loose by the water is,} \\ Q=mc\Delta t=1000*2* c*[t(f)-30] \end{gathered}](https://img.qammunity.org/qa-images/2023/formulas/physics/high-school/eiiliohig9e0iy0h39mx.png)
And for 1 liter of water the gain in heat is,
![Q=mc\Delta t=1000*1* c*[t(f)-20]](https://img.qammunity.org/qa-images/2023/formulas/physics/high-school/8lf42uzoca7veqof9dg6.png)
Now,
![\begin{gathered} Q\text{ }lose=Q\text{ }gain \\ -1000*2* c*[t(f)-30]=1000*1* c*[t(f)-20] \\ -2*[t(f)-30]=[t(f)-20] \\ -2t(f)+60=t(f)-20 \\ -3t(f)=-20-60 \\ t(f)=(80)/(3)=26.667\mathring{\text{ }}celcius \end{gathered}](https://img.qammunity.org/qa-images/2023/formulas/physics/high-school/vel2brxep70nvnn7qtt0.png)
So the final temperature of the 3 liter water = 26.667 degree cus.