Final Answer:
The density of the block of metal is approximately 318.18 grams per cubic meter.
Step-by-step explanation:
Density is defined as the mass of an object per unit volume. To calculate density, we use the formula:
![\[Density (ρ) = (Mass (m))/(Volume (V))\]](https://img.qammunity.org/2024/formulas/mathematics/high-school/vkedw1hl01toqy5giyf0sxbgzm27gml275.png)
Given that the volume of the metal block is
 , we first need to convert these units to a consistent system. Converting the volume from cubic inches to cubic meters, we use the conversion factor
, we first need to convert these units to a consistent system. Converting the volume from cubic inches to cubic meters, we use the conversion factor
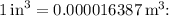
![\[Volume (V) = 13.2 \, \text{in}^3 * 0.000016387 \, \text{m}^3/\text{in}^3\]](https://img.qammunity.org/2024/formulas/mathematics/high-school/td3a0y23pdpcy6xbo3dycuns75o1qh9hnb.png)
Next, we convert the weight from pounds to grams, using the conversion factor
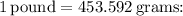
![\[Mass (m) = 5.22 \, \text{pounds} * 453.592 \, \text{grams/pound}\]](https://img.qammunity.org/2024/formulas/mathematics/high-school/eis6lvczyj8sztrt5jxza5yzr69h3meno0.png)
Now, we substitute these values into the density formula:
![\[Density (ρ) = \frac{5.22 \, \text{pounds} * 453.592 \, \text{grams/pound}}{13.2 \, \text{in}^3 * 0.000016387 \, \text{m}^3/\text{in}^3}\]](https://img.qammunity.org/2024/formulas/mathematics/high-school/62uiyc2o4s9dz9qpuxuuowkpr50pou4uic.png)
After performing the calculations, the density is approximately
 . Thus, the density of the metal block is approximately
. Thus, the density of the metal block is approximately
 , indicating the mass of 318.18 grams occupies each cubic meter of space.
, indicating the mass of 318.18 grams occupies each cubic meter of space.