Answer:
![[PCl_3]=[Cl_2]=0.068M](https://img.qammunity.org/2022/formulas/chemistry/college/lv1vrz3isrb7a7bno72iimviszut821e9c.png)
![[PCl_5]=0.385M](https://img.qammunity.org/2022/formulas/chemistry/college/28cuqbg0spv2ks6j8m3l6cuxnpf49y5nb0.png)
Step-by-step explanation:
Hello!
In this case, since the equilibrium expression for the considered equation is:
![Kc=([PCl_5])/([Cl_2][PCl_3])](https://img.qammunity.org/2022/formulas/chemistry/college/xmh60bb2cyh4i1irg7ne4oopejgqb3jxan.png)
Which can be written in terms of the reaction extent and the ICE chart and the initial concentrations of 0.453 M as shown below:
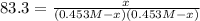
We can solve for x as follows:
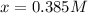
In such a way, we obtain the following concentrations at equilibrium:
![[PCl_3]=[Cl_2]=0.453M-0.385M=0.068M](https://img.qammunity.org/2022/formulas/chemistry/college/fxlkivj5p8dqaz7rdf9tzr15ui742rjiuu.png)
![[PCl_5]=0.385M](https://img.qammunity.org/2022/formulas/chemistry/college/28cuqbg0spv2ks6j8m3l6cuxnpf49y5nb0.png)
Best regards!