Answer:
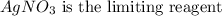
Step-by-step explanation:
Here, we want to get the limiting reactant
The limiting reactant is the reactant that would produce less amount of the solid precipitate
Firstly, we need to get the number of moles of each of the reactants
To get this, we divide their masses by the molar masses
The molar mass of magnesium chloride is 95 g/mol
The number of moles would be:
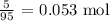
Now, from the equation of reaction, 1 mole of MgCl2 produced 2 moles of AgCl
Then: 0.053 mole of MgCl2 will produce 2 * 0.053 mol = 0.106 mol AgCl
For AgNO3, the molar mass is 170 g/mol
The number of moles would be:
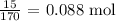
Now, looking at the equation of reaction:
2 moles of AgNO3 produce 2 moles of AgCl
0.088 mol AgNO3 will also produce 0.088 mol AgNO3
Now, looking at the values of the number of moles of AgCl produced, we can see that AgNO3 produces less of the product
This means that AgNO3 is the limiting reactant