29,769.95g of carbon dioxide can be removed.
1st) It is necessary to write the balanced chemical reaction:
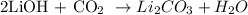
According to the balanced equation, 1 mole of carbon dioxide (CO2) can be removed by producing 1 mole of lithium carbonate (Li2CO3).
Using the molar mass of carbon dioxide and lithium carbonate we can convert moles into grams:
- CO2 molar mass: 44 g/mol
- Li2CO3 molar mass: 73.9 g/mol
With the molar mass we can see that 44g of carbon dioxide (1 mole) can be removed by producing 73.9 g of lithium carbonate.
2nd) Now we can calculate the amount of carbon dioxide that can be removed from the breathing environment if the scrubber reaction produces 50.0 Kg of Li2CO3, using a mathematical rule of three:
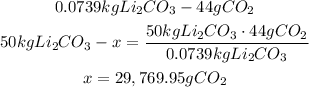
Here, it is important to convert the grams of Li2CO3 to kg before doing the calculus.
So, 29,769.95g (29.8kg) of carbon dioxide can be removed.