This question is incomplete, the complete question is;
A 1800 kg hybrid vehicle operates on ethanol and is equipped with a multipurpose motorgenerator-flywheel. When the vehicle slows or stops, 50% of the kinetic energy is recovered as electrical energy in the battery. When the IC engine is used to recharge the battery, there is a 25% efficiency of converting chemical energy in the fuel to electrical energy stored in the battery. The vehicle slows from 70 miles per hour to 20 miles per hour. Calculate: (A) Electrical energy recovered in the battery in [kJ] (B) Mass of fuel needed to store same amount of energy in the battery in [kg]
Answer:
a) Electrical energy recovered in the battery is 404.6895 kJ
b) Mass of fuel needed to store same amount of energy in the battery is 0.0606 kg
Step-by-step explanation:
Given that;
Initial speed of the vehicle V = 70 miles per hour = 31.293 m/s
Final speed of the vehicle u = 20 miles per hour = 8.941 m/s
mass of vehicle m = 1800 kg
Noe, change in kinetic energy of the vehicle will be;
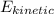 =
=
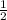 m( v² - u² )
m( v² - u² )
we substitute
=
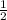 × 1800( (31.293)² - (8.941)² )
× 1800( (31.293)² - (8.941)² )
= 900( 979.2518 - 79.9414)
= 900 × 899.3104
= 809379.36 J
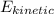 = 809.379 kJ
= 809.379 kJ
now, Electrical energy recovered in the battery when the vehicle slows will be;
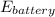 = 50% ×
= 50% ×
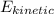
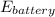 = 50/100 × 809.379 kJ
= 50/100 × 809.379 kJ
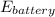 = 404.6895 kJ
= 404.6895 kJ
Therefore, Electrical energy recovered in the battery is 404.6895 kJ
b)
For this electrical energy to be obtained from fuel, the chemical energy required will be;
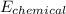 =
=
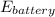 / 25%
/ 25%
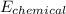 = 404.6895 kJ / 0.25
= 404.6895 kJ / 0.25
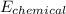 = 1618.758 kJ
= 1618.758 kJ
Heat energy released per mass of ethanol combustion
(Lower heating value of ethanol) is 26.7kJ/g
Now, the mass of fuel needed to generate 1618.758 kJ will be;
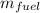 = 1618.758 kJ / 26.7kJ/g
= 1618.758 kJ / 26.7kJ/g
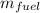 = 60.63 g
= 60.63 g
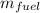 = 0.0606 kg
= 0.0606 kg
Therefore, Mass of fuel needed to store same amount of energy in the battery is 0.0606 kg