Answer: 0.1296 moles
Step-by-step explanation:
Assuming we have excess water, we only need to focus on the moles of
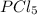 .
.
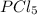 has a molar mass of 208.24 g/mol. The number of moles of
has a molar mass of 208.24 g/mol. The number of moles of
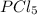 we have is
we have is
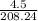 or 0.0216 moles.
or 0.0216 moles.
The two acids are HCl and H3PO4. First lets focus on the number of moles of HCl formed.
The ratio of the number of moles of HCl formed and the number of moles of
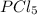 used is:
used is:
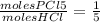 , plugging in, we get that moles of HCl = 0.108.
, plugging in, we get that moles of HCl = 0.108.
The ratio of the number of moles of H3PO4 formed and the number of moles of
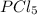 used is:
used is:
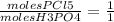 , plugging in, we get that moles of H3PO4 = 0.0216.
, plugging in, we get that moles of H3PO4 = 0.0216.
The total number of moles of acid formed is 0.0216+0.108 = 0.1296.