Final Answer:
The change in entropy ΔS for the given process is
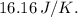
Explanation:
The change in entropy ΔS can be calculated using the formula:
![\[ΔS = nC_p \ln\left((T_f)/(T_i)\right) - nR\ln\left((V_f)/(V_i)\right)\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/jpgc0nwqkj474pbwp29y0z1wbpcfyvupb6.png) where:
where:
n = moles of gas,
 = molar heat capacity at constant pressure,
= molar heat capacity at constant pressure,
 and
and
 = initial and final temperatures,
= initial and final temperatures,
 and
and
 = initial and final volumes,
= initial and final volumes,
R = ideal gas constant
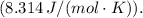
Given the conditions:
n = 3 moles,
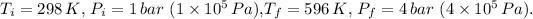
First, calculate the change in temperature
 and pressure
and pressure
 . Then, substitute these values along with the gas constant into the entropy formula. The molar heat capacity at constant pressure
. Then, substitute these values along with the gas constant into the entropy formula. The molar heat capacity at constant pressure
 for a diatomic ideal gas is 7R/2. The natural logarithm function is denoted as ln.
for a diatomic ideal gas is 7R/2. The natural logarithm function is denoted as ln.
![\[ΔS = 3 * (7R)/(2) \ln\left((596)/(298)\right) - 3R\ln\left((4 * 10^5)/(1 * 10^5)\right)\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/c7fs4ybu7xhwpxj92zamz0mrk686h89ca0.png)
After evaluating this expression, ΔS is found to be
 . This positive change in entropy indicates an increase in disorder or randomness during the heating and compression process. The system becomes more disordered as the gas molecules gain energy and occupy a smaller volume at higher temperature and pressure.
. This positive change in entropy indicates an increase in disorder or randomness during the heating and compression process. The system becomes more disordered as the gas molecules gain energy and occupy a smaller volume at higher temperature and pressure.