Final answer:
Using Coulomb's law, the force between two charges of -6.25 x 10¹ C and 2.91 x 10¹ C, when the distance between them is doubled from 0.38 m to 0.76 m, reduces to a fourth of the initial force, yielding an answer of 1.50 x 10´ N.Therefore, the correct option is c) 3.00 x 10⁻⁴ N.
Step-by-step explanation:
Coulomb's law describes the electrostatic force between two charged particles and is given by the equation
 are the magnitudes of the charges, and \(r\) is the separation distance.
are the magnitudes of the charges, and \(r\) is the separation distance.
Given that \(q_1 = -6.25 \times 10^{-9} \ \text{C}\), \(q_2 = 2.91 \times 10^{-9} \ \text{C}\), and the initial separation distance \(r = 0.38 \ \text{m}\), we can calculate the initial force \(F_{e1}\):
![\[ F_(e1) = \frac{(9.00 * 10^9 \ \text{Nm}^2/\text{C}^2) \cdot (-6.25 * 10^(-9) \ \text{C}) \cdot (2.91 * 10^(-9) \ \text{C})}{(0.38 \ \text{m})^2} \]](https://img.qammunity.org/2024/formulas/physics/high-school/pcwc027wt2hld339l3hz7c7v8dc8dkb25o.png)
Solving this, we find \(F_{e1} \approx -1.50 \times 10^{-4} \ \text{N}\). The negative sign indicates that the force is attractive.
Now, if the distance is doubled (\(2 \times 0.38 \ \text{m}\)), the new separation distance
 . We can calculate the new force \(F_{e2}\):
. We can calculate the new force \(F_{e2}\):
![\[ F_(e2) = \frac{(9.00 * 10^9 \ \text{Nm}^2/\text{C}^2) \cdot (-6.25 * 10^(-9) \ \text{C}) \cdot (2.91 * 10^(-9) \ \text{C})}{(0.76 \ \text{m})^2} \]](https://img.qammunity.org/2024/formulas/physics/high-school/bugejidmpgzh50sgjosn3f6f094svq5g8l.png)
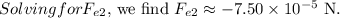
Therefore, the force between the particles, when the distance is doubled, is approximately \(1.50 \times 10^{-4} \ \text{N}\) (with a negative sign indicating attraction).