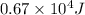Given
Amount of water,
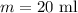
Temperature is,
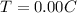
To find:
Energy required to freeze the water.
Step-by-step explanation:
The latent heat of fusion is

Converting the amount of water to kg,
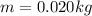
Thus the energy required is:
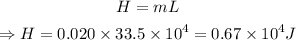
Conclusion:
The amount of energy required is