The temperature, T2, is 359°C.
1st) We need to identify the volume and the temperature of the gas in the point A (initial state of gas) and point B (final state of gas):
- Point A:
V1= 1.65x10^3 L
T1= 43°C (316K)
-Point B:
V2= 3.30x10^3 L
T2= unknown
2nd) With the Ideal Gas Law and assuming there is no change in pressure or amount of gas in the balloon, we calculate the temperature in point B with the formula that relates temperature and volume:
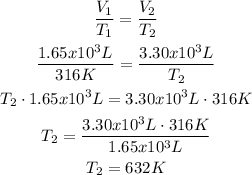
It is important to use the units of the ideal gas constant, so the units must be in Kelvin (K) and liters (L). That's why the temperature (T2) it is 316 K.
3rd) Finally, it is necessary convert the Kelvin unit into Celsiud degrees:
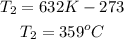
So, the temperature, T2, is 359°C.